Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) with a heterogeneous clinical course and mutational landscape. Outcomes are inferior for high risk patients (pts) treated with R-CHOP including those with a high international prognostic index (IPI), high proliferation index (Ki67), double hit/triple hit lymphoma (DH/THL), or non-germinal center (non-GC) phenotype. Several studies have suggested benefit of intensive chemotherapy (IC) with or without autologous stem cell transplantation in the upfront setting for high risk DLBCL, but this remains controversial. Many pts with high risk DLBCL are not candidates for IC (examples: R-HyperCVAD/R-methotrexate-AraC or R-CODOX-M/R-IVAC) due to advanced age, performance status and/or comorbidities, but are able to tolerate dose adjusted (DA) R-EPOCH. A recent phase III study showed no difference in event free survival (EFS) or overall survival (OS) with a median of 5 years follow-up in pts with DLBCL who received R-CHOP vs DA-R-EPOCH in the upfront setting, however subgroup analyses have not been reported (Wilson ASH 2016). Our objective was to review clinical characteristics, prognostic markers, molecular features, and outcomes of pts with high risk DLBCL who received DA-R-EPOCH at our institution.
Methods: A retrospective chart review of 59 pts diagnosed with DLBCL between 1/1/2010 and 12/31/2016 treated with DA-R-EPOCH at the John Theurer Cancer Center was performed. Pts were identified and baseline data was collected using the COTA platform which extracts and enriches electronic medical record data. Kaplan Meier survival methods were utilized. The primary endpoints were progression free survival (PFS) and OS.
Results: Median age at diagnosis was 71 years (range 32-89) and 59.3% of pts were male. Pts had high risk disease: 26 pts (44.1%) had IPI >3 and 36 pts (61.0%) had ki67 >80%. Cell of origin (COO) by Hans algorithm was consistent with GC-type in 37 pts (62.7%) and non-GC type in 22 pts (37.3%). Twelve pts (20.3%) had transformed disease: 5 (8.5%) from low grade NHL and 7 (11.9%) with Richter transformation. Among pts with full data available, 17.1% (6/35) had DH/THL by FISH and 41.2% (14/34) had double or triple expressing lymphoma (DE/TEL) by IHC. Age or performance status was the major contraindication to IC for 34 pts (57.6%) and renal or cardiac disease in 4 pts (6.7%). Almost all pts completed the planned number of DA-R-EPOCH cycles (57/59, 96.6%): 1 pt with high volume disease including leptomeningeal involvement at baseline died during cycle 1 due to sepsis and 1 pt died due to complications of a mechanical fall with head trauma after cycle 1.
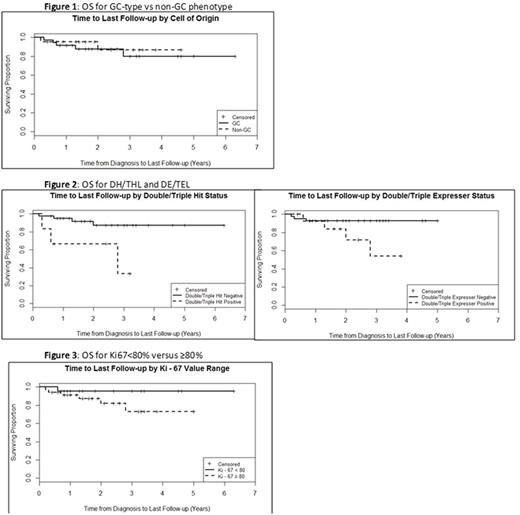
At a median of 1.9 years follow-up PFS was 72.9% and OS 88.1%. For pts with GC vs non-GC phenotype there was no difference in PFS (75.0% vs. 72.7%, p = 1.0) or OS (86.5% vs. 90.9%, p=0.70)(Figure 1). OS was inferior for 6 pts with DH/THL (50.0% vs 90.5%, p=0.03), there was a non-statistically significant trend towards inferior survival for the 14 pts with DE/TEL (71.4% vs 92.9%, p=0.057), and there was a non-statistically significant trend towards inferior PFS in both groups (50.0% vs 78.0% in DH/THL p=0.16 and 64.3% vs 78.0% in DE/TEL p=0.31)(Figure 2). Median time to progression (TTP) was not statistically different in pts with DH/THL (0.4 vs 0.7 years, p=0.058) or DE/TEL (0.7 vs 0.6 years, p=0.34). There was no difference in PFS, OS, or TTP based on p53 status, EBV status, or Ki67(Figure 3).
Conclusion: The vast majority of pts with high risk DLBCL deemed unlikely to tolerate IC were able to complete a full course of DA-R-EPOCH. OS and PFS were higher than expected compared to historical R-CHOP treatment in pts with high risk DLBCL. Though the benefit of DA-R-EPOCH has been suggested in DH/THL or DE/TEL, our experience suggests that outcomes are not improved by DA-R-EPOCH in these pts and novel approaches should be explored in this setting. There was no difference in PFS or OS based on COO, EBV status, p53 status, or Ki67 in this small cohort. Prospective data are needed to clarify the molecular and clinical features that predict benefit from DA-R-EPOCH in the upfront setting.
Leslie: seattle genetics: Speakers Bureau; celgene: Speakers Bureau; KITE pharma: Speakers Bureau. Protomastro: COTA: Employment. McNeill: seattle genetics: Speakers Bureau; celgene: Speakers Bureau; pharmacyclics: Speakers Bureau. Skarbnik: Gilead: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau; Genentech: Speakers Bureau; Novartis: Speakers Bureau; Seattle Genetics: Speakers Bureau. Goldberg: Celgene: Speakers Bureau; Bristol Myers Squibb: Research Funding, Speakers Bureau; COTA: Employment, Equity Ownership; Ariad: Speakers Bureau; Pfizer: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau; Jazz: Speakers Bureau. Pecora: Caladrius Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; COTA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Feldman: Celgene: Speakers Bureau; Kite Pharma: Speakers Bureau; Bristol-Myers Squibb: Consultancy; Janssen: Speakers Bureau; AbbVie: Speakers Bureau; Seattle Genetics: Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Speakers Bureau. Goy: Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics / J&J: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal